Experienced at being kinder to the kidneys*1
In a clinical study, AmBisome® (amphotericin B) liposome for injection demonstrated a lower incidence of nephrotoxicity than Abelcet®1
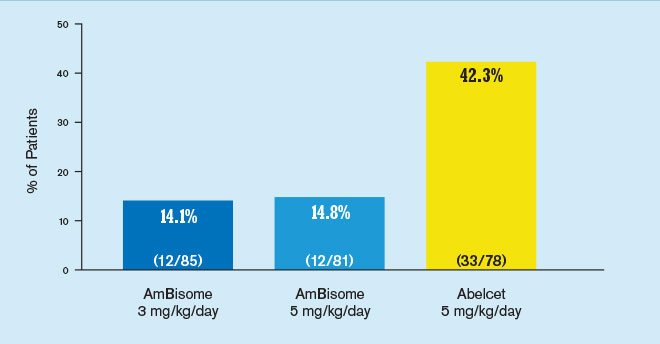
Results from a randomized, double-blind, multicenter study of 244 febrile neutropenic patients who previously received broad-spectrum antibacterial therapy, receiving either AmBisome 3 mg/kg/day (n=85) or 5 mg/kg/day (n=81), or Abelcet 5 mg/kg/day (n=78). The primary endpoint was safety and the study was not designed to draw statistically meaningful conclusions related to efficacy. Abelcet is not indicated for empiric treatment of febrile neutropenic patients.
Nephrotoxicity was defined as a serum creatinine value 2 times baseline.
Other adverse events
- In the clinical study noted above, common adverse events occurring at an incidence of 10% or more and more frequently in patients taking AmBisome compared to those taking Abelcet include: abdominal pain, sepsis, transfusion reaction, chest pain, diarrhea, bilirubinemia, edema, hypocalcemia, hypokalemia, hypomagnesemia, anxiety, confusion, headache, and rash
Incidence of infusion-related chills/rigors1
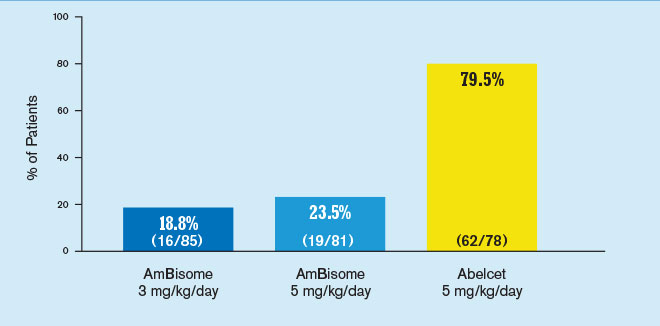
Results from a randomized, double-blind, multicenter study of 244 febrile neutropenic patients who previously received broad-spectrum antibacterial therapy, receiving either AmBisome 3 mg/kg/day (n=85) or 5 mg/kg/day (n=81), or Abelcet 5 mg/kg/day (n=78). The primary endpoint was safety and the study was not designed to draw statistically meaningful conclusions related to efficacy. Abelcet is not indicated for empiric treatment of febrile neutropenic patients.
Fewer discontinuations vs Abelcet
- Treatment discontinuations due to an adverse event were higher among patients in the Abelcet group than in the AmBisome groups
AmBisome delivered empiric antifungal power1
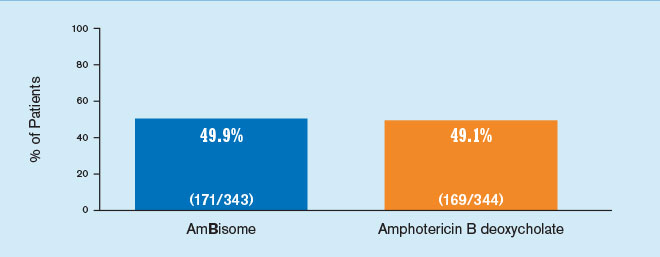
Results from a randomized, double-blind, multicenter study evaluating the efficacy of AmBisome and amphotericin B deoxycholate in 687 patients with persistent fever and neutropenia. Patients received either a mean dose of AmBisome 3 mg/kg/day (n=343) or amphotericin B deoxycholate 0.6 mg/kg/day (n=344).
Therapeutic success required: (a) resolution of fever during the neutropenic period, (b) absence of an emergent fungal infection, (c) patient survival for at least 7 days post-therapy, (d) no discontinuation of therapy due to toxicity or lack of efficacy, and (e) resolution of any study-entry fungal infection.
Important Safety Information and Indications
Contraindications
AmBisome is contraindicated in those patients who have demonstrated or have a known hypersensitivity to amphotericin B deoxycholate or any other constituents of the product, unless benefit of therapy outweighs the risk.
Warnings and Precautions
Anaphylaxis has been reported with amphotericin B–containing drugs, including AmBisome. If a severe reaction occurs, the AmBisome infusion should be immediately discontinued and the patient should not receive further infusions of AmBisome.
General: During the initial dosing period, patients should be under close observation. AmBisome has been shown to be significantly less toxic than amphotericin B deoxycholate; however, adverse events may still occur.
Laboratory Tests: Patient management should include laboratory evaluation of renal, hepatic, and hematopoietic function, and serum electrolytes (magnesium and potassium).
Drug-Laboratory Interactions: Serum Phosphate false elevation. False elevations of serum phosphate may occur when samples from patients receiving AmBisome are analyzed using the PHOSm assay.
Drug Interactions: No formal drug-interaction studies have been conducted with AmBisome. However, the following drugs are known to interact with amphotericin B and may interact with AmBisome: antineoplastic agents, corticosteroids and corticotropin (ACTH), digitalis glycosides, flucytosine, azoles (e.g. ketoconazole, miconazole, clotrimazole, fluconazole), leukocyte transfusions, other nephrotoxic medications, and skeletal muscle relaxants. (Please see Package Insert, Drug Interactions)
Adverse Reactions
The commonly reported adverse reactions across all studies with an incidence of >20% with AmBisome include: rash, hyperglycemia, hypokalemia, hypomagnesemia, diarrhea, nausea, vomiting, anemia, increased alkaline phosphatase, increased blood urea nitrogen, chills, insomnia, increased creatinine, and dyspnea.
Infusion related reactions include chills/rigors, fever, nausea, vomiting, hypertension, tachycardia, dyspnea, and hypoxia. There were a few reports of flushing, back pain with or without chest tightness, and chest pain associated with AmBisome administration; on occasion this has been severe. Where these symptoms were noted, reaction developed within a few minutes after the start of infusion and disappeared rapidly when the infusion was stopped. These symptoms do not occur with every dose and usually do not recur on subsequent administrations when the infusion rate is slowed.
Indications and Usage
AmBisome is indicated for the following:
- Empirical therapy for presumed fungal infection in febrile, neutropenic patients
- Treatment of Cryptococcal Meningitis in HIV-infected patients
- Treatment of patients with Aspergillus species, Candida species, and/or Cryptococcus species infections refractory to amphotericin B deoxycholate, or in patients where renal impairment or unacceptable toxicity precludes the use of amphotericin B deoxycholate
- Treatment of visceral leishmaniasis. In immunocompromised patients with visceral leishmaniasis treated with AmBisome, relapse rates were high following initial clearance of parasites
Please see full Prescribing Information.


